The valence electron configuration for aluminum is 3s 2 3p 1. Selenium atoms are 4-coordinate bond lengths being SeO bridging are 175 pm and 181 pm non-bridging 156 and 154 pm.
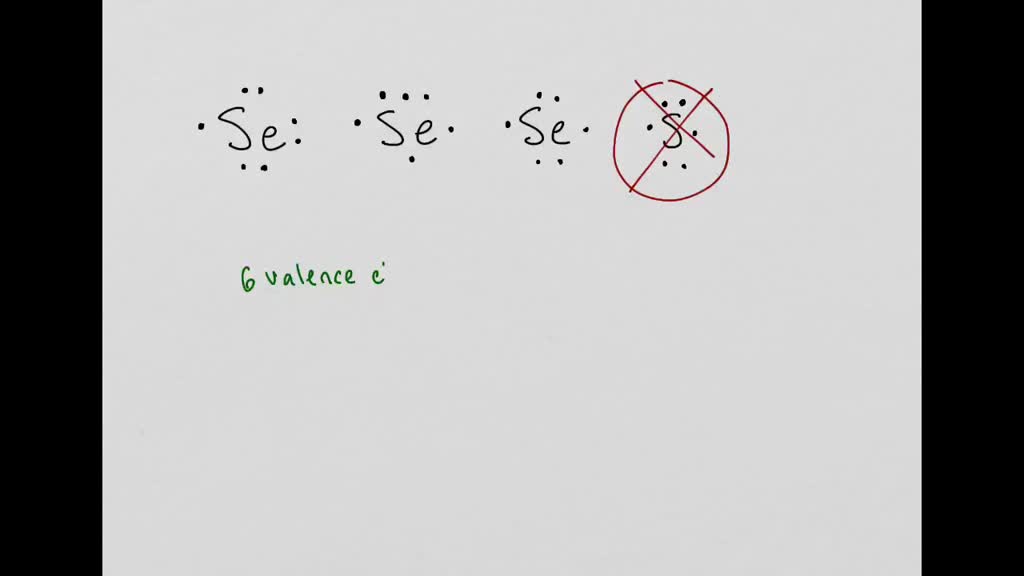
Interpret Scientific Illustrations Which Is The C
B has 3 each F has 7 and there is one extra electron.

Selenium electron dot structure. Follow some steps for drawing the lewis dot structure of SeF4. That is because S is a little more electronegative than Se and tends to work with a -2 -non real- oxidation state but it i. Um because they both have the.
Im going to start here. Is the molecule polar nonpolar or not applicable. When this occurs the addition of 2 electrons brings seleniums total electrons up to 36.
To do this selenium gains 2 electrons. Hybridization of central atom. Count the total number of electrons.
SeO3 is the chemical formula for the compound selenium trioxide. Hover for more information. Selenium bromide SeBr2 or Br2Se CID 140977 - structure chemical names physical and chemical properties classification patents literature biological.
2059 kJmole 25C. A Lewis dot structure for SeO3 is drawn with an Se in the center with two lines connecting it to two Os and one double line connecting it to an O. A step-by-step explanation of how to draw the Se Selenium Se2- Selenide ion Lewis Dot StructureFor the Se and Se 2- structure use the periodic table to.
Count total valence electron in SeF4. In the Lewis dot structure the Se represents the element selenium and each O represents oxygen. The B atom is the central atom and the F atoms are the surrounding atoms.
Write the symbol for selenium Se and put an electron pair on the top an electron pair. So it would have three dots around the symbol for aluminum two of them paired to represent the 3s electrons. The number of dots equals the number of valence electrons in the atom.
10 points A Write the COMPLETE electron configuration for selenium Se in its ground state. Burns in contact with air but is unaffected by water. Perspective drawing with bond angles.
C Write the Lewis dot structure for selenium in its ground state. In the solid phase SeO 3 consists of cyclic tetramers with an 8 membered SeO 4 ring. Draw the Lewis dot structure for Mg and Se.
DotAl nonumber The valence electron configuration for selenium is 4s 2 4p 4. These dots are arranged to the right and left and above and below the. The single dots represent one valence electron each.
Selenium tetrachloride is the inorganic compound composed with the formula SeCl 4. It is also a proven cytostatic agent slowing the growth of both hyperproliferative and normal cells in dandruff and seborrheic dermatitis. First of all determine the valence electron that is available for drawing the lewis structure of SeF4 because the lewis diagram is all about the representation of valence electrons on atoms.
Let us try these steps to determine the electron dot diagram for BF 4. Electron configuration orbital diagrams and Lewis structures of elements and ions. Lewis structures are a useful way to summarize certain information about bonding and may be thought of as electron bookkeeping.
Lewis dot structures are used to show how the atoms of a compound bond together. Selenium trichloride anion Lewis electron-dot structure. Selenium sulfide is highly active in inhibiting the growth of P.
In Lewis dot structures each dot represents an electron. Dark gray lustrous rods or dark red crystals of non-metal. A 06 micronized form of selenium sulfide is also safe and effective for dandruff.
Disolves in alkalis and concentrated HNO 3. A Lewis electron dot diagram or electron dot diagram or a Lewis diagram or a Lewis structure is a representation of the valence electrons of an atom that uses dots around the symbol of the element. Each single line indicates a single pair of bonded electrons while the double line represents two pairs of bonded electrons.
3 7 7 7 7. There is a negative sign on the species so we have an extra electron to consider. SeO 3 in the gas phase consists of tetramers and monomeric SeO 3 which is trigonal planar with an SeO bond length of 16878 pm.
B Draw the COMPLETE orbital diagram for selenium in its ground state. Put one on each side pair them after you have one on each side and put one heater. In the highest-numbered shell the n 4 shell there are six.
And here both of these show the correct electron dodge structure. Selenium has 34 electrons. But Im almost sure you meant SeS2 that is selenium disulfide not SSe2 that is sulfur diselenide.
Now another correct structure of selenium would be this. In order to become stable selenium must obtain the same configuration as xenon.